Evaluation of a device for broadcasting a recording of the maternal voice and heartbeat in hospitalized premature newborns coordinated by Dr Juliana Patkai (Service de Médecine et Réanimation Néonatales de Port-Royal, Paris)
There is some evidence to suggest that the maternal voice is an important stimulus for premature infants hospitalized in intensive care units. It could act positively to compensate for the excess sensory stimulation to which the child is exposed in these wards, resulting in an observed calming effect.
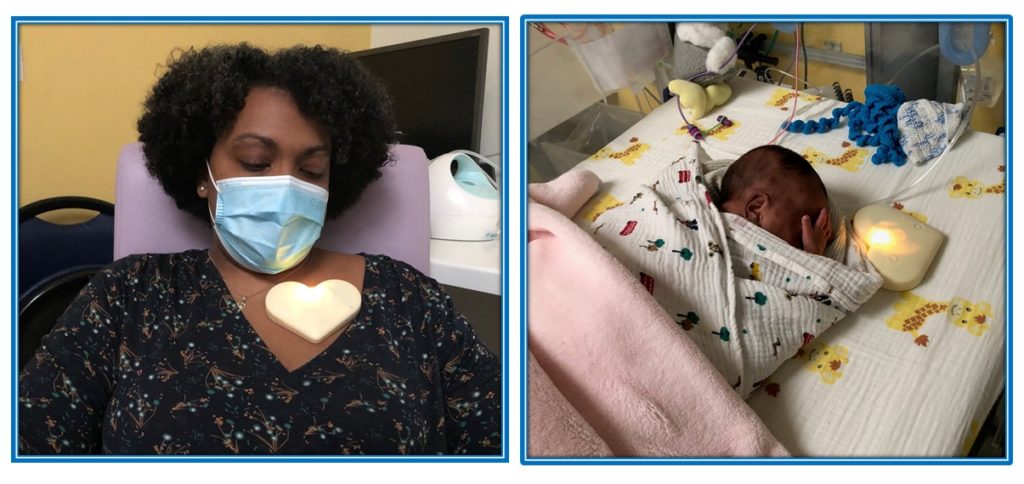
The CaliNange® device, initially designed specifically for hospital use, comes in the form of a small heart placed on the mother’s chest, without a wave and without a screen. It enables the mother’s voice to be recorded for up to 5 minutes, with the recording associated with a calming heartbeat. The sound level is limited and complies with the recommendations of scientific societies for premature or full-term newborns.
To assess the effect of Calinange®, a recording of the NIPE (Newborn Infant Parasympathetic Evaluation) index is made in the child after a treatment with or without CaliNange®.
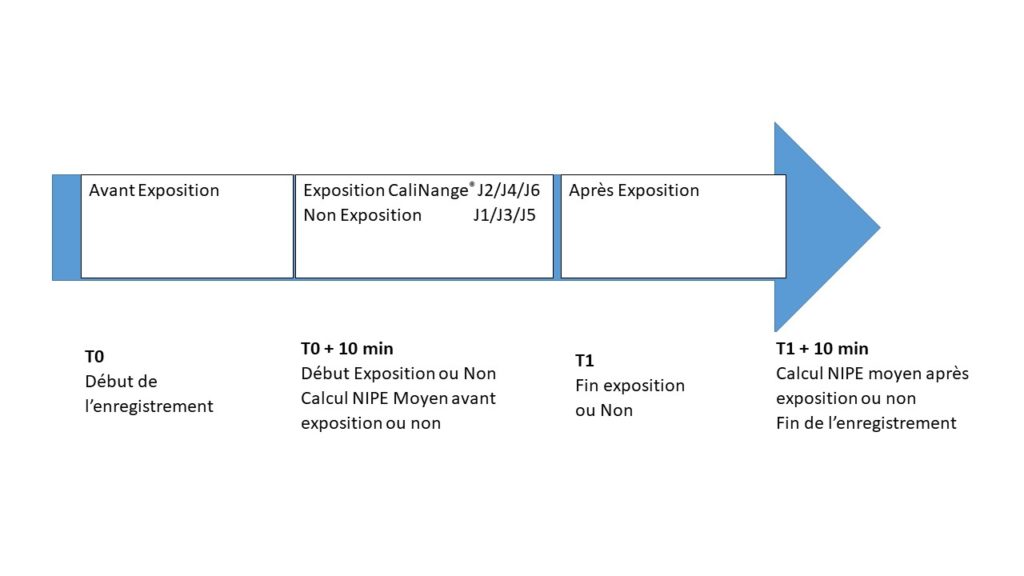
The experimental design of the CaliPrem study is:
The effect of using a CaliNange® device to broadcast the mother’s voice and heartbeat on the well-being of hospitalized premature newborns in the first days of life.
Maternal satisfaction using a satisfaction questionnaire.
The objectives of this research are to evaluate:
- The effect of using a CaliNange® device to broadcast maternal voice and heartbeat on the well-being of hospitalized premature newborns in the first days of life;
- Maternal satisfaction using a satisfaction questionnaire.
The expected benefits are :
- Improved clinical condition of the treated patient: reduced stress (heart rate (HR) variability index, and fewer critical events in the post-care period);
- Improved quality of care;
- Improved maternal experience of hospitalization.
study progress :
30 newborns were included in the neonatology department of the Port-Royal maternity hospital.
Inclusion and follow-up have been completed. Statistical analyses are in progress.
