Coordinating investigator : Dre Jeanne SIBIUDE, Hôpital Louis Mourier, Colombe, France
Scientific Director : Pr François GOFFINET, maternité Port-Royal, Hôpital Cochin, Paris, France
Manager Work-Package 1 : Dre Céline MEHATS (Institut Cochin (INSERM U1016-CNRS UMR8104-Université Paris Cité)
Manager Work-Package 2 : BForeCure
Manager Work-Package 3 : Dre Jeanne SIBIUDE (Hôpital Louis Mourier, colombes)
Sponsor : Assistance Publique – Hôpitaux de Paris
PrediMAP is a project whose objective is to develop and clinicaly evaluate an innovative in vitro diagnostic medical device (IVD-MD), to predict the risk of childbirth within 7 days of a woman’s arrival at the obstetric emergency room for preterm labor (PTL). The aim is to avoid unnecessary prenatal hospitalizations and a contrario to hospitalize pregnant women who have a high risk of spontaneous preterm delivery in order to administer beneficial treatments.
We wish to design, develop and clinically validate the predictive performance (sensitivity/specificity) of an IVD-DM, allowing rapid bedside measurement of several patented biomarkers associated with childbirth in the 7 days following their detection.
The biomarkers ‘results, obtained by the multiplex immuno-PCR technique, will be integrated into an algorithm with clinical and ultrasound data (cervical length) to obtain a personalized prediction of the risk of premature birth in 7 days, for women consulting for suspicion of PTL.
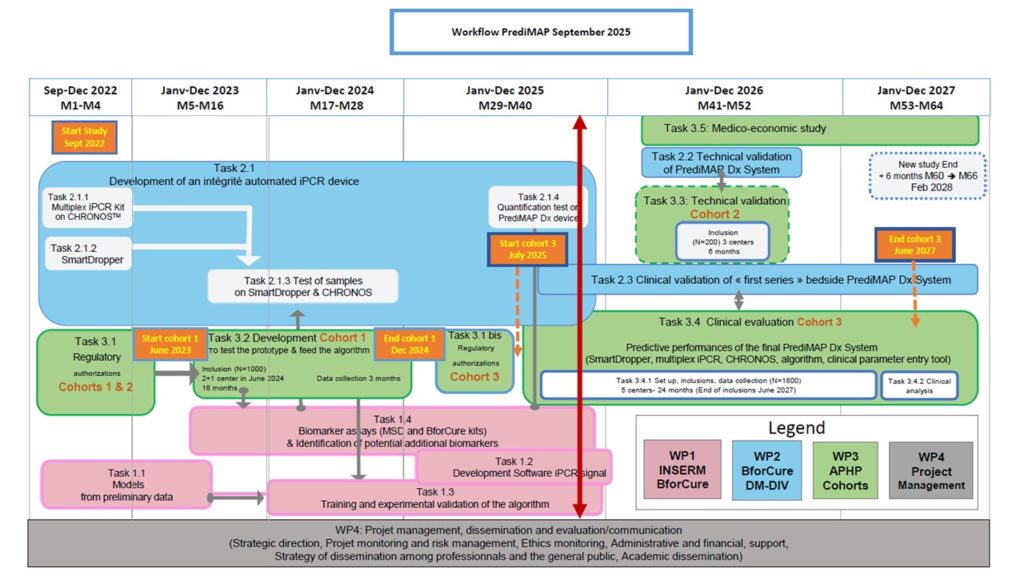
The PrediMAP project, started in 2022, takes place in 2 steps, with 3 cohorts of patients.
In 2024, an initial database of 500 patients from cohort 1 enabled the development of a first
algorithm. Cohort 1 ended in December 2024 with 894 patients included. The rate of inclusion was in line with expectations, with a higher than expected rate of women giving birth within 7 days (7% versus 5%), which provides reassurance regarding the algorithm’s construction.
Biomarker assays are performed by the U1016 research team, and aliquots allowed BFC to test the DM-DIV interface for assay reliability. Mono-plex immuno-PCR kits have already been developed and continue to be improved for multiplexing. In addition, it has been decided to centralize the biomarker measurements of cohort 3, initially planned for the emergency department, to simplify the process and guarantee its progress. These adjustments will make it possible to maintain the project’s objective within the allotted time frame.
The second phase of the study was conducted at Port-Royal, Louis Mourier, Armand Trousseau, Robet Debré, and Saint Joseph Paris centers. The first inclusion in cohort 3 took place on June 30, 2025.
At the end of August , 2025, there are 119 inclusions in cohort 3.